Volume -
3 , - : 47-51 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Effect of Oral Menthol on Antioxidant Status of Polycystic Ovarian Syndrome-Induced Wister Rats. 3 https://doi.org/10.58209/gmjm.4.2.47
URL: http://daneshafarand.org/article-1-250-en.html
URL: http://daneshafarand.org/article-1-250-en.html
| Abstract (HTML) (2626 Views)
Full-Text: (1127 Views)
Introduction
Polycystic ovarian syndrome (PCOS), so called Stein-Leventhal syndrome has been known to have metabolic and reproductive endocrinopathy disorders [1]. Prevalence of PCOS has been reported to be 5-20% in reproductive age [2]. Patients with PCOS are highly sensitive to some diseases and disorders including obesity, insulin resistance, type II diabetes, cardiovascular disease, infertility, malignancy, and psychological disorders [2].
The exact etiology of the PCOS is still unknown, but could be attributed to complex interactions among different factors including genetic, environmental, and behavioral factors. Anxiety, depression, and poor quality of life have been diagnosed in patients with PCOS [3]. Insulin has been known as one of the atherogenic hormones [4] which along with hyperinsulinemia could participate in the development of diabetes, hypertension, and dyslipidemia, which is often combined with elevated total cholesterol and low-density lipoprotein (LDL), triglyceride (TG), and reduced high-density lipoprotein (HDL) levels in patients with PCOS [5]. Hyperinsulinemia has been known to have the ability to promote ovarian androgen overproduction [6]. Dyslipidemia and sex steroids have also been known to have important effects on cardiovascular diseases [7]. Elevated oxidative stress and reduced antioxidant capacity can contribute to the increasing risk of cardiovascular disease in patients with PCOS, in addition to insulin resistance, hypertension, central obesity, and dyslipidemia [7].
The allopathic drugs have commonly been used to treat the PCOS, which include clomiphene citrate, metformin, letrozole, tamoxifene and troglitazone. These drugs have been known to have severe side effects including hot flushes, arthritis, joint or muscle pain and psychological side effects [8]. Since conventional medicine can have side effects, the alternative drugs such as herbal medicines and their derivations can play a significant role. Menthol is one natural cyclic monoterpene alcohol which is found in Mentha species. Menthol is also one of the most important constituents of some essential oils including eucalyptus, lemongrass, and palmarosa [9]. Rozza et al. [10] have shown that menthol can have a gastro protective role against ethanol-induced gastric ulcers and treatment with elevated levels of the anti-inflammatory cytokine IL-10. Menthol has also been known to have antioxidant properties [11].
It seems that menthol has improved antioxidant and lipid profile in animals with PCOS. This study was therefore conducted to evaluate the effects of oral administration of menthol on blood biochemical parameters and antioxidant in mice with PCOS.
Materials and Methods
Preparation of menthol
Menthol oil (Barij Essence; Iran) was crystallized
through chilling in the +14, +10 and –5°C for 8 hours each, respectively, by using sealed plastic containers in freezers. Menthol crystals were isolated from peppermint essential oil because dementholized peppermint essential oil can still contain certain amounts of menthol, racemic and isomenthols and menthone. In order to recover the menthol crystals and to remove the menthone, it had to be treated with 8g boric acid in distillation flask for a period of 3 hours. The rest of the distillation containing borates of menthol were saponified using steam distillation on 70g of 15% NaOH solution, and finally crystals were separated and dried in 26°C and production was investigated.
Animals
Fifty female Wistar rats, 13-15 weeks of age, weighing 170±20g, were purchased from Pasture Institute, Tehran-Iran. To adapt, the female rats were grouped into 5 groups in the controlled temperature of 22-24°C and a lighting diet of 12h light:12h darkness cycle. Food and water were ad libitum supplied.
Induction of PCOS and treatments
The Wistar rats were distributed into five groups:
1) Control group (Control) or healthy rats that did not receive any menthol;
2) PCOS group (PCOS) that did not receive any menthol;
3) PCOS induced rats that received 2mg/kg of body weight of menthol (PCOS-2)
4) PCOS induced rats that received 4mg/kg of body weight of menthol (PCOS-4)
5) PCOS induced rats that received 6mg/kg of body weight of menthol (PCOS-6).
In the PCOS induction phase, rats were induced with PCOS by applying 5mg estradiol valerate, as reported by previous studies [12]. Wistar rats were treated with menthol for 28 days. Body weight was recorded in days 1, 14 and 28 post-induction.
Blood sampling and biochemical analyses
At the end of the trial, all the Wistar rats were anesthetized by using ketamine/xylazine HCl (75/10mg/kg intraperitoneally). The collected blood samples from the aorta with anticoagulant were centrifuged for 12min in 3500rpm/min to achieve the plasma and stored in -20°C until biochemical analysis for investigation of glucose, insulin, total cholesterol, triglycerides, HDL-C and LDL-C. The plasma concentrations of glucose, insulin, total cholesterol, triglycerides, HDL-C and LDL-C were investigated by using Pars Azmoon commercial Kits.
Ferric-reducing ability of plasma (FRAP)
FRAP reagent was produced and heated in 37°C while also a mixture of the following solutions was applied [14]: 1) 0.3M sodium acetate buffer solution (pH 3.6), 2) 10mM 2,4,6-tripyridyl-1-5-triazine in 40mM HCl solution, and 3) 20 mM FeCl3 solution at the ratio of 10:1:1 (v/v/v). A level of plasma (10μL) was incubated along along with 90μL of FRAP reagent in a micro plate for 30 minutes in room temperature in the dark. The level of absorbance of the mixture was assessed in the wavelength of 595nm by a spectrophotometer. The levels of FRAP values were measured by a calibration standard curve of FeSO4 (0-2000μM).
Advanced oxidation protein product (AOPP)
Samples were made as follows: in a tube, 20μL of plasma from each rat was diluted into 100μL in phosphate-buffered saline by the inclusion of 10μL of 1.16M KI and 20μL of absolute acetic acid. The absorbance of the reaction mixture was rapidly read by a SpectraMax1601 spectrophotometer (Sunnyvale; USA) in 340nm against a blank, having 100μL of phosphate-buffered saline, 20μL of acetic acid, and 10μL of KI solution. As the linear range of chloramine-T absorbance in 340nm is between 0 and 100μM, AOPP concentrations were reported in μM chloramine-T equivalents. All evaluations were conducted simultaneously.
Total oxidation status (TOS)
The plasma concentration of TOS was evaluated by a colorimetric measurement procedure. In order to evaluate the TOS, 225μL of Reagent 1 (xylenol orange 150μM, NaCl 140mM, and glycerol 1.35M in 25 mMH2SO4 solution, pH 1.75) was mixed with 35μL of plasma sample, and the absorbance of each sample was investigated spectrophotometrically in 560nm as a sample blank. Then, 11μL of Reagent 2 (ferrous ion (5mM) and o-dianisidine (10mM) in 25mM H2SO4 solution) was added to the mixture within 3-4 min. The last absorbance was investigated in 560nm. The assay was calibrated by using H2O2, and the results have been reported in terms of micromolar H2O2 equivalent per liter (μmol H2O2equiv/L). The detection limit of the procedure was investigated by evaluating the zero calibrator 10 times.
Statistical analysis
The statistical analyses were conducted via GraphPad Prism software. The analysis of variance (ANOVA) was used to compare among the groups, and post hoc (Tukey) was used to compare the groups. Significance was considered in p<0.05.
Findings
Body weight
Effects of different levels of menthol on body weight of Wistar rats are presented in Figure 1. As the results indicate, body weight was not influenced by experimental treatments in day 1 (p>0.05). There was no significant difference between control and PCOS or control PCOS in day 1 (p>0.05). In days 14 and 28, induction of PCOS significantly increased body weight, so control PCOS rats showed higher body weight in comparison to control group (p<0.05). Oral gavage of menthol could significantly decrease body weight. There was positive correlation between body weight and level of PCOS (p<0.05), so higher levels of menthol could significantly decrease body weight (p<0.05).
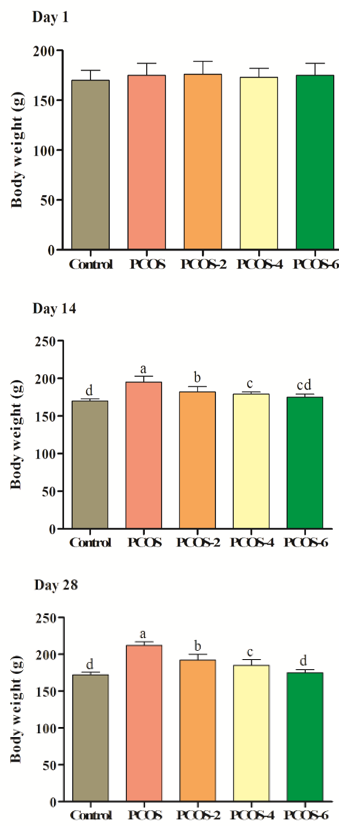
Figure 1. Effects of different levels of menthol (2, 4 & 6mg/kg) on body weight (g) in rats with PCOS. Control=control standard without PCOS, PCOS= PCOS control without menthol, PCOS-2, 4 & 6= PCOS rats treated with 2, 4 and 6 mg/kg menthol. Superscripts (a-e) show significant differences at p<0.05.
Blood biochemical parameters
Induction of PCOS could significantly increase LDL-C, cholesterol, triglycerides, insulin and glucose but also decrease HDL-C (control versus PCOS group (p<0.05). However, oral administration of menthol could significantly increase HDL-C and decrease LDL-C, cholesterol, triglycerides, insulin and glucose (p<0.05). The best responses were observed in highest levels of menthol (6mg/kg; Table 1).
Table 1. Effects of different levels of menthol on blood biochemical parameters in rats with PCOS

Antioxidant status
The induction of PCOS increased oxidation in terms of FRAP, AOPP and TOS (p<0.05; control vs. PCOS). Oral treatment with menthol significantly improved antioxidant levels in comparison to PCOS group. As levels of menthol were increased, oxidation status was decreased respectively (Figure 2).
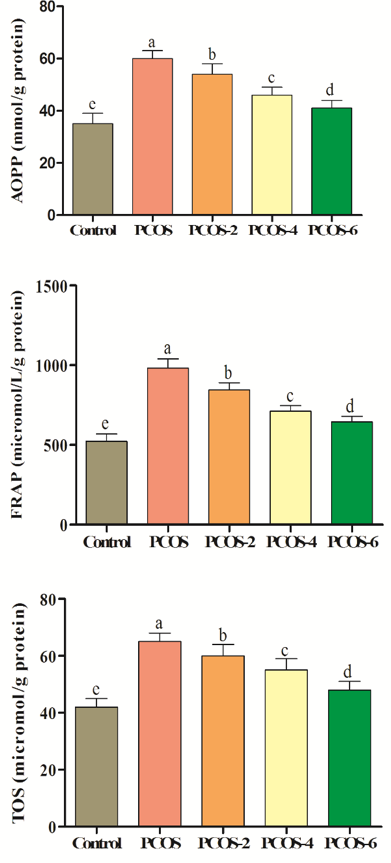
Figure 2. Effects of different levels of menthol (2, 4 & 6mg/kg) on antioxidant status in rats with PCOS. Control=control standard without PCOS, PCOS= PCOS control without menthol, PCOS-2, 4 & 6= PCOS rats treated with 2, 4 and 6mg/kg menthol. Superscripts (a-e) show significant differences at p<0.05.
Discussion
Body weight significantly increased in PCOS rats compared to the control group. Kim et al. [14] showed in their study that increased body weight in PCOS rats induced with dehydroepiandrosterone. The difference between control group and PCOS group could be attributed to inferring obesity [15], and diets can have a major role in weight gain in animals with PCOS. It has been accepted that high-fiber diet and low-fat diets could decrease body weight [16]. In the current study, menthol comprises very small portion of the diet and thus cannot have a role due to low fat levels. It seems that menthol decreases oxidation and subsequent dyslipidemia, and dyslipidemia could have a major role in increased body weight. Maintained body weight could be attributed to antioxidant status.
Hyperglycemia was observed in rats with PCOS. PCOS has been reported as one metabolic disorder related with type 2 diabetes mellitus [17] and it is caused by hyperglycemia in initial phases which leads to insulin resistance. Similarly, other studies have reported hyperglycemia in Letrozole induced PCOS rats [18]. Hyperinsulinemia was also observed in our study and it is known to have the ability to promote ovarian androgen overproduction [6]. Menthol decreased levels of insulin and glucose, and it seemed that menthol increases sensitivity to insulin and decreases glucose.
Dyslipidemia was also observed in rats with PCOS (control vs. PCOS). Imbalanced lipid profile has been related to hyper-androgenemia [19, 20]. Lipid peroxidation has been known as one of the markers for oxidative tissue damage. It also induces free radical damage to the components of cell membrane that cause cell necrosis and inflammation [21]. Some studies have reported oxidative stress as one of the pathological factors for PCOS [22, 23].
Elevated oxidant levels could change the stereo diagnosis in ovaries which could be attributed to raised androgen production and polycystic ovaries [22]. In the present study, menthol improves lipid profile by antioxidant system, and our findings for oxidation system supported our hypotheses. In sum, it could be stated that antioxidant activity of menthol prevents lipid peroxidation and blood parameters could be considered as index for such action. Future studies are needed to evaluate the effects of menthol. Therefore, we recommend the use of menthol for treatment of PCOS as a novel agent.
Conclusion
PCOS has negative effects on almost all blood parameters as well as the antioxidant status. Oral administration of menthol improves blood parameters through antioxidant system.
Acknowledgements: None declared by the authors.
Ethical Permissions: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Authors’ Contribution: All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Funding/Support: None declared by the authors.
Polycystic ovarian syndrome (PCOS), so called Stein-Leventhal syndrome has been known to have metabolic and reproductive endocrinopathy disorders [1]. Prevalence of PCOS has been reported to be 5-20% in reproductive age [2]. Patients with PCOS are highly sensitive to some diseases and disorders including obesity, insulin resistance, type II diabetes, cardiovascular disease, infertility, malignancy, and psychological disorders [2].
The exact etiology of the PCOS is still unknown, but could be attributed to complex interactions among different factors including genetic, environmental, and behavioral factors. Anxiety, depression, and poor quality of life have been diagnosed in patients with PCOS [3]. Insulin has been known as one of the atherogenic hormones [4] which along with hyperinsulinemia could participate in the development of diabetes, hypertension, and dyslipidemia, which is often combined with elevated total cholesterol and low-density lipoprotein (LDL), triglyceride (TG), and reduced high-density lipoprotein (HDL) levels in patients with PCOS [5]. Hyperinsulinemia has been known to have the ability to promote ovarian androgen overproduction [6]. Dyslipidemia and sex steroids have also been known to have important effects on cardiovascular diseases [7]. Elevated oxidative stress and reduced antioxidant capacity can contribute to the increasing risk of cardiovascular disease in patients with PCOS, in addition to insulin resistance, hypertension, central obesity, and dyslipidemia [7].
The allopathic drugs have commonly been used to treat the PCOS, which include clomiphene citrate, metformin, letrozole, tamoxifene and troglitazone. These drugs have been known to have severe side effects including hot flushes, arthritis, joint or muscle pain and psychological side effects [8]. Since conventional medicine can have side effects, the alternative drugs such as herbal medicines and their derivations can play a significant role. Menthol is one natural cyclic monoterpene alcohol which is found in Mentha species. Menthol is also one of the most important constituents of some essential oils including eucalyptus, lemongrass, and palmarosa [9]. Rozza et al. [10] have shown that menthol can have a gastro protective role against ethanol-induced gastric ulcers and treatment with elevated levels of the anti-inflammatory cytokine IL-10. Menthol has also been known to have antioxidant properties [11].
It seems that menthol has improved antioxidant and lipid profile in animals with PCOS. This study was therefore conducted to evaluate the effects of oral administration of menthol on blood biochemical parameters and antioxidant in mice with PCOS.
Materials and Methods
Preparation of menthol
Menthol oil (Barij Essence; Iran) was crystallized
through chilling in the +14, +10 and –5°C for 8 hours each, respectively, by using sealed plastic containers in freezers. Menthol crystals were isolated from peppermint essential oil because dementholized peppermint essential oil can still contain certain amounts of menthol, racemic and isomenthols and menthone. In order to recover the menthol crystals and to remove the menthone, it had to be treated with 8g boric acid in distillation flask for a period of 3 hours. The rest of the distillation containing borates of menthol were saponified using steam distillation on 70g of 15% NaOH solution, and finally crystals were separated and dried in 26°C and production was investigated.
Animals
Fifty female Wistar rats, 13-15 weeks of age, weighing 170±20g, were purchased from Pasture Institute, Tehran-Iran. To adapt, the female rats were grouped into 5 groups in the controlled temperature of 22-24°C and a lighting diet of 12h light:12h darkness cycle. Food and water were ad libitum supplied.
Induction of PCOS and treatments
The Wistar rats were distributed into five groups:
1) Control group (Control) or healthy rats that did not receive any menthol;
2) PCOS group (PCOS) that did not receive any menthol;
3) PCOS induced rats that received 2mg/kg of body weight of menthol (PCOS-2)
4) PCOS induced rats that received 4mg/kg of body weight of menthol (PCOS-4)
5) PCOS induced rats that received 6mg/kg of body weight of menthol (PCOS-6).
In the PCOS induction phase, rats were induced with PCOS by applying 5mg estradiol valerate, as reported by previous studies [12]. Wistar rats were treated with menthol for 28 days. Body weight was recorded in days 1, 14 and 28 post-induction.
Blood sampling and biochemical analyses
At the end of the trial, all the Wistar rats were anesthetized by using ketamine/xylazine HCl (75/10mg/kg intraperitoneally). The collected blood samples from the aorta with anticoagulant were centrifuged for 12min in 3500rpm/min to achieve the plasma and stored in -20°C until biochemical analysis for investigation of glucose, insulin, total cholesterol, triglycerides, HDL-C and LDL-C. The plasma concentrations of glucose, insulin, total cholesterol, triglycerides, HDL-C and LDL-C were investigated by using Pars Azmoon commercial Kits.
Ferric-reducing ability of plasma (FRAP)
FRAP reagent was produced and heated in 37°C while also a mixture of the following solutions was applied [14]: 1) 0.3M sodium acetate buffer solution (pH 3.6), 2) 10mM 2,4,6-tripyridyl-1-5-triazine in 40mM HCl solution, and 3) 20 mM FeCl3 solution at the ratio of 10:1:1 (v/v/v). A level of plasma (10μL) was incubated along along with 90μL of FRAP reagent in a micro plate for 30 minutes in room temperature in the dark. The level of absorbance of the mixture was assessed in the wavelength of 595nm by a spectrophotometer. The levels of FRAP values were measured by a calibration standard curve of FeSO4 (0-2000μM).
Advanced oxidation protein product (AOPP)
Samples were made as follows: in a tube, 20μL of plasma from each rat was diluted into 100μL in phosphate-buffered saline by the inclusion of 10μL of 1.16M KI and 20μL of absolute acetic acid. The absorbance of the reaction mixture was rapidly read by a SpectraMax1601 spectrophotometer (Sunnyvale; USA) in 340nm against a blank, having 100μL of phosphate-buffered saline, 20μL of acetic acid, and 10μL of KI solution. As the linear range of chloramine-T absorbance in 340nm is between 0 and 100μM, AOPP concentrations were reported in μM chloramine-T equivalents. All evaluations were conducted simultaneously.
Total oxidation status (TOS)
The plasma concentration of TOS was evaluated by a colorimetric measurement procedure. In order to evaluate the TOS, 225μL of Reagent 1 (xylenol orange 150μM, NaCl 140mM, and glycerol 1.35M in 25 mMH2SO4 solution, pH 1.75) was mixed with 35μL of plasma sample, and the absorbance of each sample was investigated spectrophotometrically in 560nm as a sample blank. Then, 11μL of Reagent 2 (ferrous ion (5mM) and o-dianisidine (10mM) in 25mM H2SO4 solution) was added to the mixture within 3-4 min. The last absorbance was investigated in 560nm. The assay was calibrated by using H2O2, and the results have been reported in terms of micromolar H2O2 equivalent per liter (μmol H2O2equiv/L). The detection limit of the procedure was investigated by evaluating the zero calibrator 10 times.
Statistical analysis
The statistical analyses were conducted via GraphPad Prism software. The analysis of variance (ANOVA) was used to compare among the groups, and post hoc (Tukey) was used to compare the groups. Significance was considered in p<0.05.
Findings
Body weight
Effects of different levels of menthol on body weight of Wistar rats are presented in Figure 1. As the results indicate, body weight was not influenced by experimental treatments in day 1 (p>0.05). There was no significant difference between control and PCOS or control PCOS in day 1 (p>0.05). In days 14 and 28, induction of PCOS significantly increased body weight, so control PCOS rats showed higher body weight in comparison to control group (p<0.05). Oral gavage of menthol could significantly decrease body weight. There was positive correlation between body weight and level of PCOS (p<0.05), so higher levels of menthol could significantly decrease body weight (p<0.05).

Figure 1. Effects of different levels of menthol (2, 4 & 6mg/kg) on body weight (g) in rats with PCOS. Control=control standard without PCOS, PCOS= PCOS control without menthol, PCOS-2, 4 & 6= PCOS rats treated with 2, 4 and 6 mg/kg menthol. Superscripts (a-e) show significant differences at p<0.05.
Blood biochemical parameters
Induction of PCOS could significantly increase LDL-C, cholesterol, triglycerides, insulin and glucose but also decrease HDL-C (control versus PCOS group (p<0.05). However, oral administration of menthol could significantly increase HDL-C and decrease LDL-C, cholesterol, triglycerides, insulin and glucose (p<0.05). The best responses were observed in highest levels of menthol (6mg/kg; Table 1).
Table 1. Effects of different levels of menthol on blood biochemical parameters in rats with PCOS

Antioxidant status
The induction of PCOS increased oxidation in terms of FRAP, AOPP and TOS (p<0.05; control vs. PCOS). Oral treatment with menthol significantly improved antioxidant levels in comparison to PCOS group. As levels of menthol were increased, oxidation status was decreased respectively (Figure 2).

Figure 2. Effects of different levels of menthol (2, 4 & 6mg/kg) on antioxidant status in rats with PCOS. Control=control standard without PCOS, PCOS= PCOS control without menthol, PCOS-2, 4 & 6= PCOS rats treated with 2, 4 and 6mg/kg menthol. Superscripts (a-e) show significant differences at p<0.05.
Discussion
Body weight significantly increased in PCOS rats compared to the control group. Kim et al. [14] showed in their study that increased body weight in PCOS rats induced with dehydroepiandrosterone. The difference between control group and PCOS group could be attributed to inferring obesity [15], and diets can have a major role in weight gain in animals with PCOS. It has been accepted that high-fiber diet and low-fat diets could decrease body weight [16]. In the current study, menthol comprises very small portion of the diet and thus cannot have a role due to low fat levels. It seems that menthol decreases oxidation and subsequent dyslipidemia, and dyslipidemia could have a major role in increased body weight. Maintained body weight could be attributed to antioxidant status.
Hyperglycemia was observed in rats with PCOS. PCOS has been reported as one metabolic disorder related with type 2 diabetes mellitus [17] and it is caused by hyperglycemia in initial phases which leads to insulin resistance. Similarly, other studies have reported hyperglycemia in Letrozole induced PCOS rats [18]. Hyperinsulinemia was also observed in our study and it is known to have the ability to promote ovarian androgen overproduction [6]. Menthol decreased levels of insulin and glucose, and it seemed that menthol increases sensitivity to insulin and decreases glucose.
Dyslipidemia was also observed in rats with PCOS (control vs. PCOS). Imbalanced lipid profile has been related to hyper-androgenemia [19, 20]. Lipid peroxidation has been known as one of the markers for oxidative tissue damage. It also induces free radical damage to the components of cell membrane that cause cell necrosis and inflammation [21]. Some studies have reported oxidative stress as one of the pathological factors for PCOS [22, 23].
Elevated oxidant levels could change the stereo diagnosis in ovaries which could be attributed to raised androgen production and polycystic ovaries [22]. In the present study, menthol improves lipid profile by antioxidant system, and our findings for oxidation system supported our hypotheses. In sum, it could be stated that antioxidant activity of menthol prevents lipid peroxidation and blood parameters could be considered as index for such action. Future studies are needed to evaluate the effects of menthol. Therefore, we recommend the use of menthol for treatment of PCOS as a novel agent.
Conclusion
PCOS has negative effects on almost all blood parameters as well as the antioxidant status. Oral administration of menthol improves blood parameters through antioxidant system.
Acknowledgements: None declared by the authors.
Ethical Permissions: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Authors’ Contribution: All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Funding/Support: None declared by the authors.
Subject:
Biotechnology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






