Volume -
3 , - : 53-57 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Modulation Effects of Carvacrol on Inflammatory and Antioxidant System’s Gene Expression of Diabetic Rats. 3 https://doi.org/10.58209/gmjm.4.2.53
URL: http://daneshafarand.org/article-1-251-en.html
URL: http://daneshafarand.org/article-1-251-en.html
| Abstract (HTML) (2050 Views)
Full-Text: (907 Views)
Introduction
Diabetes mellitus is a complex disease because it is a metabolic disorder that is involved with oxidation and inflammation [1]. It has been accepted that inflammation has roles in type 1 and type 2 diabetes mellitus [2, 3]. Studies have shown increased inflammatory markers in patients with diabetes [4]. Diabetic nephropathy has been known as one of the most common complications in diabetes and it is also main reason for end-stage renal disease in all over world [5].
Glucose is entered into cells in independent form of insulin and accumulated glucose increases inflammation, cell oxidation and apoptosis [6]. Pathogenesis of diabetic nephropathy could be due to binary of hemodynamic changes and excessive hyperglycemia, resulting in increased inflammation and oxidative stress [7, 8]. Increased oxidative stress and formation of pro-inflammatory cytokines happens during diabetes [9]. During Inflammation, inflammatory reactions raise levels of tumor necrosis factor-α (TNF-alpha), interlukin-1β (IL-1β) and IL-6 that interact with proteins [10]. The TNF-α has been known as an inflammatory cytokine that initiates inflammation. The IL-1β is a pro-inflammatory cytokine that calls of neutrophils into the region of infection [11].
On the other hand, oxidative stress is known to have excessive importance in diabetes. There is an imbalance between the formation and neutralization of reactive oxygen species (ROS) including highly reactive hydroxyl radicals’ superoxide anion, peroxyl radicals, singlet oxygen, peroxynitrite, and hydrogen peroxide. Enzymatic and non-enzymatic antioxidants help to treat and/or prevent the diabetes and its related complications [12]. Synthetic drugs have also been used to treat the diabetes, but its use has faced with limitations due to side effects. Plant derivations have also been used to treat the different diseases. Carvacrol, one monoterpenic phenol, that is extensively existed in some species including Origanum, Satureja, Thymbra, Thymus, and Corydothymus [13]. Carvacrol has been reported to have some pharmacological features including antioxidant [14], anti-inflammatory [15], antitumor [16], and antimicrobial [17] activity.
It seems that carvacrol could improve diabetes due to its antioxidant and anti-inflammatory. We aim to introduce the carvacrol as a novel agent for treatment of diabetes. This study was thus conducted to evaluate the effects of carvacrol on inflammatory and antioxidant responses of diabetic rats.
Materials and Methods
Materials
Carvacrol was prepared from Fluka, Chemika, Sigma-Aldrich (St Quentin Fallavier, France) in purity of 95%. Streptozotocin was also prepared from Sigma-Aldrich Company. All other chemical agents used were analytical grade and prepared from standard commercial suppliers.
Animals
Adult male Wistar rats (220±20g) were from the Pasture Institute, Tehran-Iran. Animals were kept in lighting program of 12h/12h light/dark cycle. Animals had ad libitum access to food and water. All the used principles were approved by the Guide for the Care and Use of Laboratory Animals, USA, 1986.
Induction of diabetes
A single dose of streptozotocin (55mg/kg body weight) in 0.1M cold citrate buffer (pH 4.5) was intra peritoneal administrated to fasted rats. Control animals received citrate buffer alone. In overnight, animals consumed 5% glucose solution in order to overcome on hypoglycemia. Seventy-two hours after administration of streptozotocin, glucose was assessed by glucometer and rats with glucose concentrations>300mg/dL were used as diabetic and studied. Animals were grouped one week after administration of streptozotocin and lasted for 7 days.
Experimental design
The animals (n=50) were grouped into five treatments, each comprising of ten animals:
Group 1) Control animals giving 0.1M citrate buffer (pH=4.5).
Group 2) Diabetic controls.
Group 3) Diabetic animals given carvacrol (5 mg/kg Body weight/day) in neutral sterile olive oil solution oral gavage.
Group 4) Diabetic animals given carvacrol (10 mg/kg Body weight/day) in neutral sterile olive oil solution oral gavage.
Group 5) Diabetic animals given carvacrol (15 mg/kg Body weight/day) in neutral sterile olive oil solution oral gavage.
Evaluation of blood glucose
In day 7 of trial, the Wistar rats were fasted at over night, anaesthetized and sacrificed, by cervical dislocation. The blood samples was gathered in tubes with EDTA for investigation of plasma glucose.
Assessment of antioxidant activity
After killing rats, the livers were removed and washed with isotonic saline. The liver tissue was homogenated and used in 5% (w/v) potassium phosphate buffer (0.1M, pH 7.4) by a homogenizer. The homogenate sample was then centrifuged in 16000×g for 20min in order to remove the nuclei and cell debris. The supernatant was applied for measurement of lipid peroxidation and antioxidant activity as previously reported [18]. In order to evaluate the malondialdehyde (MDA), TBARS contents of the samples were measured from a standard curve by 1,1,3,3-tetramethoxypropane.Catalase activity was measured by the molar extinction coefficient of 43.6M-1cm-1 for H2O2 and reported as μmol H2O2 consumed/min per milligram of protein. To evaluate the superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities, diagnostic kits of RANSOD and ANSEL (Randox: UK) were used and the values were reported as unit/mg protein. Protein level of the samples was assessed as reported by Bradford [19].
Real-time RT-PCR for IL-1β, IL-6 and TNF-α
Parts of liver samples were used to evaluate the Real-time RT-PCR for IL-1β, IL-6 and TNF-α. It was investigated as reported by Kha et al. [20]. The primers sequences were IL-1β, forward (5′-CACCTTCTTTTCCTTCATCTTTG-3′) and reverse (5′-GTCGTTGCTTGTCTCTCCTTGTA -3′), IL-6, forward (5′-TGATGGATGCTTCCAAACTG-3′) and reverse (5′-GAGCATTGGAAGTTGGGGTA-3′), TNF-α, forward (5′-ACTGAACTTCGGGGTGATTG -3′) and reverse (5′-GCTTGGTGGTTTGCTACGAC-3′) and GADPH forward (5′-GTATTGGGCGCCTGGTCACC-3′) and reverse (5′-CGCTCCTGGAAGATGGTGATGG-3′).
Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA) and post-hoc was conducted by the Dunnett multiple comparison tests by the SPSS 20 software and graphs were illustrated by Graph Pad software.
Findings
Plasma glucose
The plasma concentration of glucose was significantly increased in diabetic Wistar rats in comparison to control group (p<0.05). Oral administration of carvacrol could significantly decrease glucose concentration and the best responses were observed in Wistar rats given with highest levels of carvacrol (p<0.05; Figure 1).
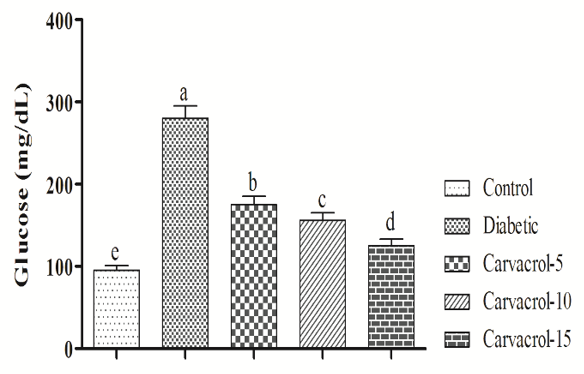
Figure 1. Effects of different levels of carvacrol on plasma glucose (mg/dL) in diabetic rats
Antioxidant status
Diabetic control rats showed lower levels for antioxidant enzymes and higher levels for MDA in comparison to healthy control (p<0.05). Oral administration of carvacrol could alleviate negative effects of diabetes on antioxidant status, so that we did not observe significant difference between healthy control rats and those received carvacrol in level of 15mg/kg (p>0.05; Table 1).
Pro-inflammatory cytokines gene expression
Diabetes increased levels of pro-inflammatory cytokines but oral administration of carvacrol especially in higher levels could alleviate effects of diabetes on pro-inflammatory cytokines (Figure 2).
Table 1. Effects of different levels of carvacrol on antioxidant parameters in diabetic rats
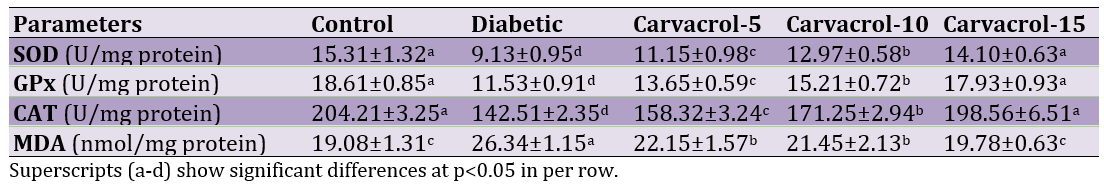
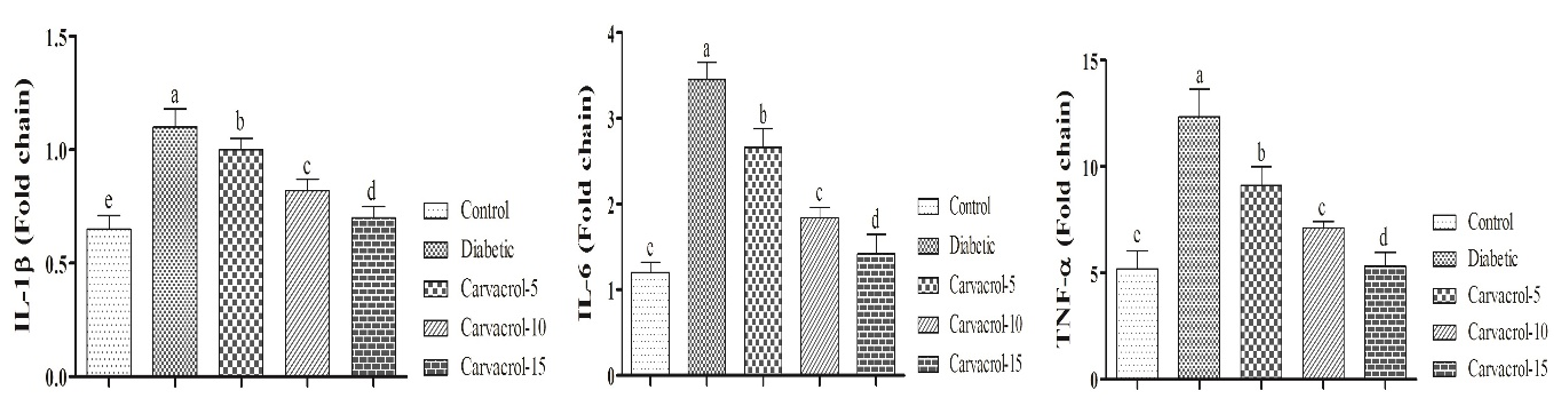
Figure 2. Effects of different levels of carvacrol on pro-inflammatory cytokines in diabetic rats
Discussion
Administration of streptozotocin could significantly increase levels of glucose and MDA. MDA is end product for lipid peroxidation. There is a correlation exists between lipid peroxidation and increased glucose. Manna et al. [21] showed increased lipid peroxidation in tissue that attributed it to increased blood glucose. Lipid peroxidation has been known during diabetes mellitus which is one key feature in patients with diabetes mellitus. The free radical and reactive oxygen species modulate in the different types of diseases such as diabetes. The free radicals could have important role in diabetes mellitus [22]. Lipid peroxidation is known to have risks for body. Increased lipid peroxidation in the tissues of diabetic rats could be attributed to increased TBARS and hydroperoxides in the tissues [23]. With regards to increased glucose, it has been reported a positive correlation between hyperglycemia and inflammation [24]. It means that inflammation stimulates insulin resistance in the physiological level [25, 26]. Inflammation is involved in progression of diabetes and increases hyperglycemia. Oral administration of carvacrolcould significantly decreases levels of glucose. Ezhumalai et al. [27] have reported that administration of carvacrol significantly decreased level of glucose and glycosylated hemoglobin. Improved levels of glucose could be attributed to antioxidant status and inflammatory responses. Results showed that inflammatory responses and antioxidant status were significantly improved in Wistar rats fed with higher levels of carvacrol. It has been reported decreased activities of enzymatic antioxidants during diabetes, and it could be attributed to a number of deleterious effects due to the increased free radicals [28]. The SOD and CAT are known as two key enzymes that remove the toxic free radicals induced by STZ. The activities of SOD and CAT were decreased in liver tissue of diabetic rats [29]. SOD is involved in conversion of the superoxide radicals into H2O2 and molecular oxygen and also maintains the tissues from highly reactive hydroxyl radical by catalyzing the decreased hydrogen peroxides [29]. In the present study, carvacrol improved glucose level and MDA by improving level of antioxidants. Similarly, Deng et al. [30] reported that the use of carvacrol could prevent increased oxidative stress, also TNF-α andNF-κB signaling in diabetic rats [31, 32]. As mentioned earlier that diabetes is related with inflammation and thus our result shows that carvacrol decreases inflammation in diabetic rat model.
Conclusion
Carvacrol helps animals with diabetes in terms of hyperglycemia, inflammation and oxidation and higher levels of carvacrol show anti-inflammatory and antioxidant properties.
Acknowledgements: : None declared by the authors.
Ethical Permissions: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Authors’ Contribution: All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Funding/Support: None declared by the authors.
Diabetes mellitus is a complex disease because it is a metabolic disorder that is involved with oxidation and inflammation [1]. It has been accepted that inflammation has roles in type 1 and type 2 diabetes mellitus [2, 3]. Studies have shown increased inflammatory markers in patients with diabetes [4]. Diabetic nephropathy has been known as one of the most common complications in diabetes and it is also main reason for end-stage renal disease in all over world [5].
Glucose is entered into cells in independent form of insulin and accumulated glucose increases inflammation, cell oxidation and apoptosis [6]. Pathogenesis of diabetic nephropathy could be due to binary of hemodynamic changes and excessive hyperglycemia, resulting in increased inflammation and oxidative stress [7, 8]. Increased oxidative stress and formation of pro-inflammatory cytokines happens during diabetes [9]. During Inflammation, inflammatory reactions raise levels of tumor necrosis factor-α (TNF-alpha), interlukin-1β (IL-1β) and IL-6 that interact with proteins [10]. The TNF-α has been known as an inflammatory cytokine that initiates inflammation. The IL-1β is a pro-inflammatory cytokine that calls of neutrophils into the region of infection [11].
On the other hand, oxidative stress is known to have excessive importance in diabetes. There is an imbalance between the formation and neutralization of reactive oxygen species (ROS) including highly reactive hydroxyl radicals’ superoxide anion, peroxyl radicals, singlet oxygen, peroxynitrite, and hydrogen peroxide. Enzymatic and non-enzymatic antioxidants help to treat and/or prevent the diabetes and its related complications [12]. Synthetic drugs have also been used to treat the diabetes, but its use has faced with limitations due to side effects. Plant derivations have also been used to treat the different diseases. Carvacrol, one monoterpenic phenol, that is extensively existed in some species including Origanum, Satureja, Thymbra, Thymus, and Corydothymus [13]. Carvacrol has been reported to have some pharmacological features including antioxidant [14], anti-inflammatory [15], antitumor [16], and antimicrobial [17] activity.
It seems that carvacrol could improve diabetes due to its antioxidant and anti-inflammatory. We aim to introduce the carvacrol as a novel agent for treatment of diabetes. This study was thus conducted to evaluate the effects of carvacrol on inflammatory and antioxidant responses of diabetic rats.
Materials and Methods
Materials
Carvacrol was prepared from Fluka, Chemika, Sigma-Aldrich (St Quentin Fallavier, France) in purity of 95%. Streptozotocin was also prepared from Sigma-Aldrich Company. All other chemical agents used were analytical grade and prepared from standard commercial suppliers.
Animals
Adult male Wistar rats (220±20g) were from the Pasture Institute, Tehran-Iran. Animals were kept in lighting program of 12h/12h light/dark cycle. Animals had ad libitum access to food and water. All the used principles were approved by the Guide for the Care and Use of Laboratory Animals, USA, 1986.
Induction of diabetes
A single dose of streptozotocin (55mg/kg body weight) in 0.1M cold citrate buffer (pH 4.5) was intra peritoneal administrated to fasted rats. Control animals received citrate buffer alone. In overnight, animals consumed 5% glucose solution in order to overcome on hypoglycemia. Seventy-two hours after administration of streptozotocin, glucose was assessed by glucometer and rats with glucose concentrations>300mg/dL were used as diabetic and studied. Animals were grouped one week after administration of streptozotocin and lasted for 7 days.
Experimental design
The animals (n=50) were grouped into five treatments, each comprising of ten animals:
Group 1) Control animals giving 0.1M citrate buffer (pH=4.5).
Group 2) Diabetic controls.
Group 3) Diabetic animals given carvacrol (5 mg/kg Body weight/day) in neutral sterile olive oil solution oral gavage.
Group 4) Diabetic animals given carvacrol (10 mg/kg Body weight/day) in neutral sterile olive oil solution oral gavage.
Group 5) Diabetic animals given carvacrol (15 mg/kg Body weight/day) in neutral sterile olive oil solution oral gavage.
Evaluation of blood glucose
In day 7 of trial, the Wistar rats were fasted at over night, anaesthetized and sacrificed, by cervical dislocation. The blood samples was gathered in tubes with EDTA for investigation of plasma glucose.
Assessment of antioxidant activity
After killing rats, the livers were removed and washed with isotonic saline. The liver tissue was homogenated and used in 5% (w/v) potassium phosphate buffer (0.1M, pH 7.4) by a homogenizer. The homogenate sample was then centrifuged in 16000×g for 20min in order to remove the nuclei and cell debris. The supernatant was applied for measurement of lipid peroxidation and antioxidant activity as previously reported [18]. In order to evaluate the malondialdehyde (MDA), TBARS contents of the samples were measured from a standard curve by 1,1,3,3-tetramethoxypropane.Catalase activity was measured by the molar extinction coefficient of 43.6M-1cm-1 for H2O2 and reported as μmol H2O2 consumed/min per milligram of protein. To evaluate the superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities, diagnostic kits of RANSOD and ANSEL (Randox: UK) were used and the values were reported as unit/mg protein. Protein level of the samples was assessed as reported by Bradford [19].
Real-time RT-PCR for IL-1β, IL-6 and TNF-α
Parts of liver samples were used to evaluate the Real-time RT-PCR for IL-1β, IL-6 and TNF-α. It was investigated as reported by Kha et al. [20]. The primers sequences were IL-1β, forward (5′-CACCTTCTTTTCCTTCATCTTTG-3′) and reverse (5′-GTCGTTGCTTGTCTCTCCTTGTA -3′), IL-6, forward (5′-TGATGGATGCTTCCAAACTG-3′) and reverse (5′-GAGCATTGGAAGTTGGGGTA-3′), TNF-α, forward (5′-ACTGAACTTCGGGGTGATTG -3′) and reverse (5′-GCTTGGTGGTTTGCTACGAC-3′) and GADPH forward (5′-GTATTGGGCGCCTGGTCACC-3′) and reverse (5′-CGCTCCTGGAAGATGGTGATGG-3′).
Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA) and post-hoc was conducted by the Dunnett multiple comparison tests by the SPSS 20 software and graphs were illustrated by Graph Pad software.
Findings
Plasma glucose
The plasma concentration of glucose was significantly increased in diabetic Wistar rats in comparison to control group (p<0.05). Oral administration of carvacrol could significantly decrease glucose concentration and the best responses were observed in Wistar rats given with highest levels of carvacrol (p<0.05; Figure 1).

Figure 1. Effects of different levels of carvacrol on plasma glucose (mg/dL) in diabetic rats
Antioxidant status
Diabetic control rats showed lower levels for antioxidant enzymes and higher levels for MDA in comparison to healthy control (p<0.05). Oral administration of carvacrol could alleviate negative effects of diabetes on antioxidant status, so that we did not observe significant difference between healthy control rats and those received carvacrol in level of 15mg/kg (p>0.05; Table 1).
Pro-inflammatory cytokines gene expression
Diabetes increased levels of pro-inflammatory cytokines but oral administration of carvacrol especially in higher levels could alleviate effects of diabetes on pro-inflammatory cytokines (Figure 2).
Table 1. Effects of different levels of carvacrol on antioxidant parameters in diabetic rats


Figure 2. Effects of different levels of carvacrol on pro-inflammatory cytokines in diabetic rats
Discussion
Administration of streptozotocin could significantly increase levels of glucose and MDA. MDA is end product for lipid peroxidation. There is a correlation exists between lipid peroxidation and increased glucose. Manna et al. [21] showed increased lipid peroxidation in tissue that attributed it to increased blood glucose. Lipid peroxidation has been known during diabetes mellitus which is one key feature in patients with diabetes mellitus. The free radical and reactive oxygen species modulate in the different types of diseases such as diabetes. The free radicals could have important role in diabetes mellitus [22]. Lipid peroxidation is known to have risks for body. Increased lipid peroxidation in the tissues of diabetic rats could be attributed to increased TBARS and hydroperoxides in the tissues [23]. With regards to increased glucose, it has been reported a positive correlation between hyperglycemia and inflammation [24]. It means that inflammation stimulates insulin resistance in the physiological level [25, 26]. Inflammation is involved in progression of diabetes and increases hyperglycemia. Oral administration of carvacrolcould significantly decreases levels of glucose. Ezhumalai et al. [27] have reported that administration of carvacrol significantly decreased level of glucose and glycosylated hemoglobin. Improved levels of glucose could be attributed to antioxidant status and inflammatory responses. Results showed that inflammatory responses and antioxidant status were significantly improved in Wistar rats fed with higher levels of carvacrol. It has been reported decreased activities of enzymatic antioxidants during diabetes, and it could be attributed to a number of deleterious effects due to the increased free radicals [28]. The SOD and CAT are known as two key enzymes that remove the toxic free radicals induced by STZ. The activities of SOD and CAT were decreased in liver tissue of diabetic rats [29]. SOD is involved in conversion of the superoxide radicals into H2O2 and molecular oxygen and also maintains the tissues from highly reactive hydroxyl radical by catalyzing the decreased hydrogen peroxides [29]. In the present study, carvacrol improved glucose level and MDA by improving level of antioxidants. Similarly, Deng et al. [30] reported that the use of carvacrol could prevent increased oxidative stress, also TNF-α andNF-κB signaling in diabetic rats [31, 32]. As mentioned earlier that diabetes is related with inflammation and thus our result shows that carvacrol decreases inflammation in diabetic rat model.
Conclusion
Carvacrol helps animals with diabetes in terms of hyperglycemia, inflammation and oxidation and higher levels of carvacrol show anti-inflammatory and antioxidant properties.
Acknowledgements: : None declared by the authors.
Ethical Permissions: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Authors’ Contribution: All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Funding/Support: None declared by the authors.
Subject:
Biotechnology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






